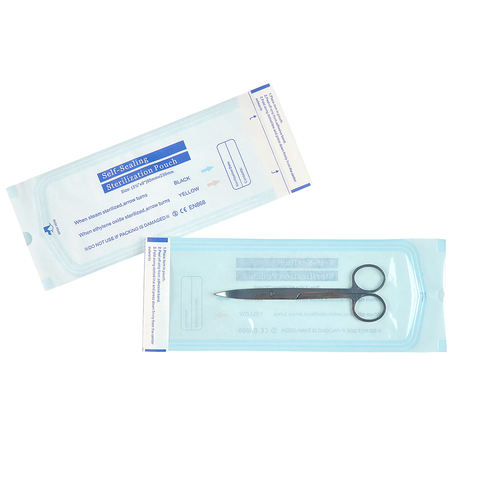
Dental-Use Medical Autoclave Self-Sealing Sterilization Pouches represent the cornerstone of modern dental instrument aseptic processing. These purpose-engineered pouches provide a secure, validated, and user-friendly system for packaging, sterilizing, storing, and aseptically presenting individual instruments or small sets, directly supporting compliance with the highest standards of dental infection control.
Technical Specifications & Material Science: The pouch is a sophisticated dual-component laminate. The front side is a transparent, medical-grade polyester or polypropylene plastic film. This layer is impervious to microorganisms and liquids, providing a durable, see-through barrier that allows for immediate visual identification of contents without compromising sterility. The back side is a sterilization-grade porous paper or a spunbond-meltblown non-woven material. This critical layer is engineered with precisely sized pores that permit the full penetration of steam (or other sterilization agents like ethylene oxide) during the autoclave cycle while acting as an effective bacterial filter post-sterilization to maintain package integrity. The defining feature is the integrated pressure-sensitive, self-sealing adhesive strip along the open end. This thermo-tacky adhesive requires no external heat sealer; a firm press creates an immediate, tamper-evident, and secure seal. Each pouch incorporates internal chemical process indicators (typically ink strips that turn dark upon exposure to heat and steam) and external areas for recording sterilization date, cycle number, and contents.
Industry Standards & Compliance: This product is a regulated medical device. It must conform to the rigorous ISO 11607-1 & -2 standards for “Packaging for terminally sterilized medical devices,” which specify requirements for materials, sterile barrier systems, and packaging processes. Compliance with EN 868 series (Packaging materials and systems) is essential for the European market. The porous side must pass specific tests for air permeability and bacterial barrier properties. Production occurs in an ISO 13485 certified facility, and the final product is validated for use in standard dental steam autoclave cycles (e.g., 121°C/250°F or 134°C/273°F).
Application Scenarios: These pouches are indispensable in every dental practice for the daily sterilization of critical and semi-critical items. They are used for packaging: high-speed and low-speed handpieces, prophylaxis angles, ultrasonic scaler inserts, surgical forceps and elevators, dental mirrors, composite placement instruments, and endodontic files. Their role extends from routine examination kits to specialized surgical instruments, ensuring every item that enters a patient’s mouth is delivered from a sterile barrier.

Usage Guidelines: The protocol is straightforward but must be meticulous: 1) Ensure instruments are clean, dry, and cool. 2) Select an appropriately sized pouch, allowing ample space (at least 2.5 cm or 1 inch) around the instrument. 3) Place the instrument inside, ensuring sharp points do not pierce the pouch. 4) Remove the protective liner from the adhesive area, fold over, and apply firm, even pressure along the entire seal width. 5) Label the pouch with the load date, sterilizer ID, and contents using an indelible marker. 6) Place pouches in the autoclave with the paper/porous side facing the same direction (usually upward or toward the chamber wall) to ensure proper steam penetration. 7) After the cycle, allow pouches to dry completely inside the autoclave before handling to prevent wicking contamination. The sterile package remains intact until deliberately torn open at the point of use.
Market Value Proposition: For the dental practice, these pouches are more than a consumable; they are a fundamental risk-mitigation tool. Their use is auditable evidence of compliance with CDC guidelines and dental board regulations for instrument processing. The self-sealing feature eliminates the capital expense and maintenance of a heat sealer, reducing clutter and simplifying training. For distributors, sterilization pouches are a high-frequency, high-volume “replenishment” product with unwavering demand. Offering a range of sizes and potentially private-label (OEM) options builds deep brand integration into the clinic’s core workflow. Supplying a reliable, compliant pouch makes you a partner in patient safety, securing a recurring revenue stream tied directly to the practice’s patient volume.